Major Side Effects of Impurities in Fluphenazine Tablets
Fluphenazine is a powerful antipsychotic medication used to treat chronic psychiatric disorders such as schizophrenia. While effective when manufactured correctly, the presence of impurities in Fluphenazine tablets can turn this therapeutic agent into a serious health hazard. These impurities—whether arising from raw materials, degradation, or manufacturing errors—can intensify adverse reactions or introduce new toxic effects altogether.
1.Severe Neurotoxicity
Impurities may affect the central nervous system, leading to symptoms like seizures, confusion, or intense agitation. These effects can be life-threatening, especially in patients with pre-existing neurological conditions.
2. Extrapyramidal Symptoms (EPS)
Fluphenazine already carries a risk of tremors, muscle rigidity, and involuntary movements. Impurities can worsen or unpredictably trigger these side effects, making the drug intolerable or dangerous.
3. Cardiotoxicity
Contaminants in Fluphenazine tablets may lead to irregular heart rhythms (arrhythmias), QT prolongation, or even sudden cardiac arrest in vulnerable individuals.
4.Liver and Kidney Damage
Toxic impurities can place added stress on the liver and kidneys, leading to long-term organ damage or failure. Patients with compromised detox systems are especially at risk.
5. Hypersensitivity Reactions
Unknown impurities can provoke allergic or anaphylactic reactions, including rashes, difficulty breathing, or swelling of the face and throat.
6. Potential Carcinogenic or Genotoxic Effects
Some impurities—especially those that result from degradation—may have mutagenic or carcinogenic properties, increasing the long-term risk of cancer.
What Are Impurities in Pharmaceuticals
Impurities are unintended substances that may be present in active pharmaceutical ingredients (APIs) or finished drug products. These can arise from:
- Raw materials
- Synthesis processes
- Degradation over time
- Storage conditions
Identifying and managing these impurities ensures:
- Patient safety
- Regulatory compliance
Why Impurity Profiling Matters
Impurity profiling refers to the detection, identification, and analysis of unwanted substances. This process is essential for several reasons:
- Safety First: Some impurities can be toxic. Profiling helps identify and minimize these risks.
- Regulatory Approval: Authorities like the FDA and EMA require detailed impurity profiles for drug approval.
- Quality Control: Monitoring ensures consistency and reliability across production batches.
OMCHEM LABS: A Global Leader in Impurity Profiling
Founded with a vision to elevate pharmaceutical standards, OMCHEM LABS has become a trusted name in impurity profiling. Backed by state-of-the-art R&D facilities and experienced scientists, the company delivers:
- Technical excellence
- Regulatory insight
- Global support
They specialize in:
- Custom Impurity Synthesis
– Tailored impurity development for antibiotics, steroids, and both chiral and achiral drugs. - Certified Reference Standards (CRS)
– High-purity benchmarks for analytical testing with a vast impurity catalog and growing chemical database.
Analytical Expertise at Its Best
OMCHEM LABS provides advanced analytical capabilities to ensure precision in impurity analysis, including:
- Method Development & Validation
– Creating and validating techniques for trace-level impurity detection. - Stability Testing
– Studying how drug compounds degrade and the impurities they form over time. - Structure Elucidation
– Using advanced instrumentation to determine the molecular structure of unknown impurities.
These services help companies maintain global compliance and ensure safe, high-quality pharmaceutical products.
Supporting Regulatory Success
Navigating global pharmaceutical regulations can be complex. OMCHEM LABS supports clients with:
- Regulatory documentation & dossier preparation
- Submissions for Drug Master Files (DMFs) and Abbreviated New Drug Applications (ANDAs)
- Expertise to meet international regulatory expectations
A Global Partner You Can Trust
OMCHEM LABS holds various international certifications and has been audited by leading agencies, reflecting their dedication to:
- Quality
- Compliance
- Global pharmaceutical standards
During the manufacturing and formulation of Fluphenazine Dihydrochloride, a variety of impurities may form either as process-related by-products or through degradation mechanisms.

Fluphenazine Dihydrochloride / Fluphenazine Decanoate EP Impurity B
CAS No.: 146-56-5
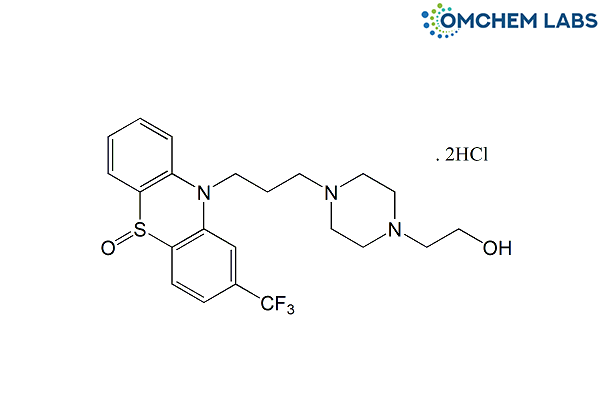
Fluphenazine Dihydrochloride EP Impurity A / Fluphenazine Decanoate EP Impurity A
CAS No.: 1674-76-6

Fluphenazine Dihydrochloride EP Impurity B
CAS No.: 1476-79-5

Fluphenazine Dihydrochloride EP Impurity D
CAS No.: 2376-89-8

Fluphenazine Dihydrochloride EP Impurity E
CAS No.: 58-39-9

Fluphenazine N-Deshydroxyethyl Impurity
CAS No.: 2804-16-2
Final Thoughts
Impurity profiling is not just a regulatory checkbox—it’s a vital part of pharmaceutical safety, effectiveness, and trust. With:
- Unmatched expertise
- Global presence
- Relentless focus on quality
OMCHEM LABS continues to lead the way in impurity profiling and reference standard development for the pharmaceutical industry.
